You may report adverse events to BioMarin at drugsafety@bmrn.com. You are encouraged to report negative adverse events of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
This website is intended only for United States healthcare professionals; if you are not a United States healthcare professional, please visit www.BioMarin.com
This website was developed and funded by BioMarin and is intended for healthcare professionals only. It offers a non-exhaustive selection of materials, including but not limited to publications, presentations, posters and educational materials, to support scientific exchange.
Clinical study results reflect knowledge at the study’s completion and may not be current. Review the methodology, limitations, and authors’ financial disclosures for details.
The information on this website is not medical advice and should not replace independent medical judgment or further research. Content on investigational therapeutics or uses of approved products does not confirm safety or efficacy. BioMarin does not endorse off-label use of its medicines.
As product information may vary by country, please refer to your local health authority regulations and respective approved product information for indications and safety details.
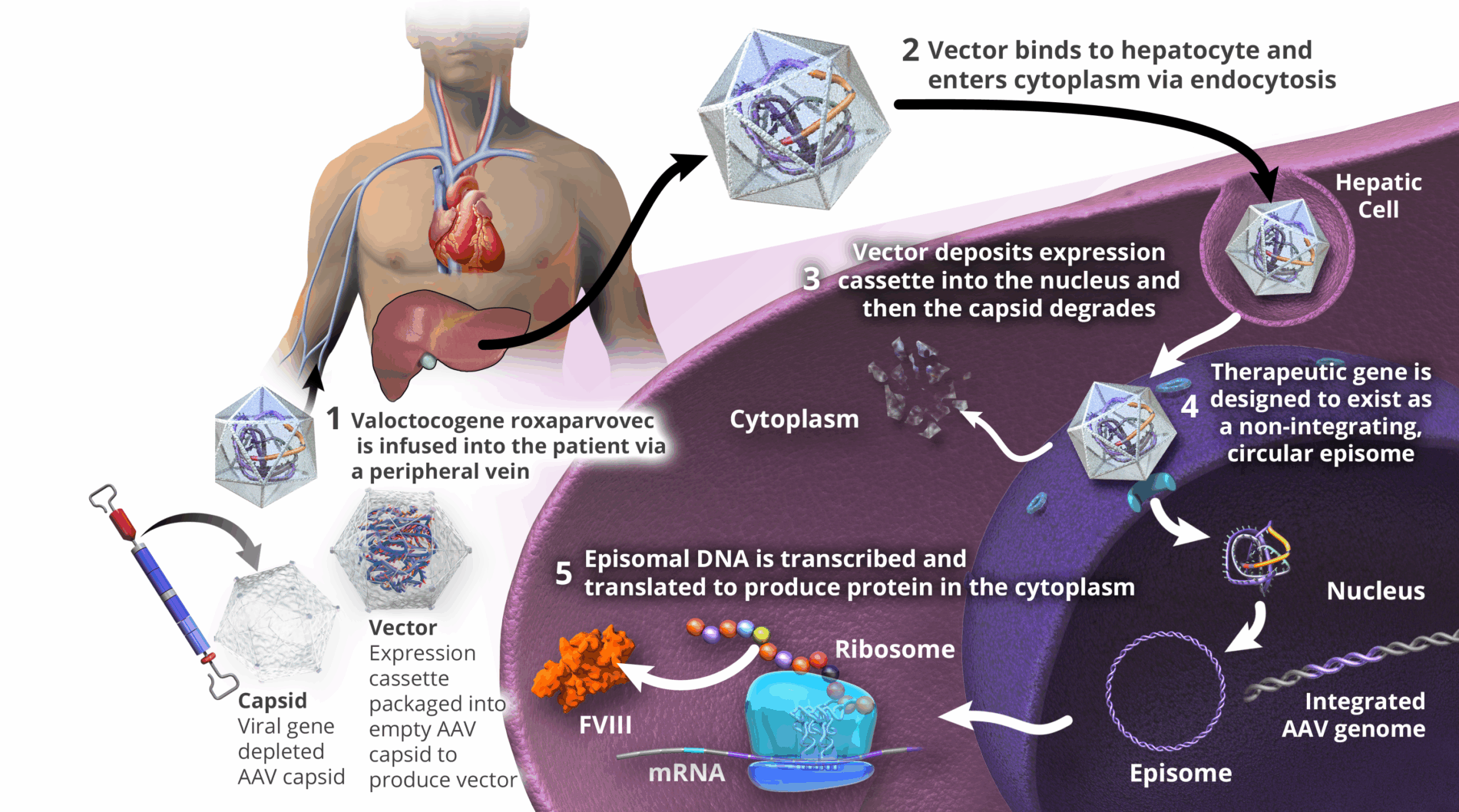
Valoctocogene roxaparvovec is designed to deliver therapeutic transgene to the hepatocyte nucleus, where it is expected to persist as extra-chromosomal episomes; however, valoctocogene roxaparvovec can insert into the DNA of human body cells.7,17,18 The clinical relevance of individual vector insertional events is not known to date.
You may report adverse events to BioMarin at drugsafety@bmrn.com. You are encouraged to report negative adverse events of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.